Strictly diastereocontrolled photocyclodimerization of 2-anthracenecarboxylates tethered to cyclic tetrasaccharides†
Abstract
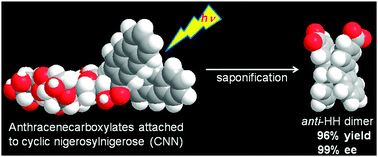
Photocyclodimerization of 2-anthracenecarboxylate tethered to a cyclic nigerosylnigerose scaffold gave a single chiral cyclodimer (out of two achiral and two chiral stereoisomers) in 99% optical and 96% chemical yields, achieving the ultimate stereocontrol of the supramolecular photochirogenesis in aqueous solution at 25 °C.

 Please wait while we load your content...
Please wait while we load your content...